Омекшавање воде
1 additions to document , most recent about 8 years ago
| When | Why |
|---|---|
| Nov-03-17 | Појашњење теме |
0 General Document comments
0 Sentence and Paragraph comments
0 Image and Video comments
Omekšavanje vode









Omekšavanje vode je postupak kojim se iz tvrde vode uklanjaju kalcijumovi i magnezijumovi joni. Najčešće se vrši jonskim izmenjivačima koji uklonjene jone zamenjuju natrijumovim jonima. Voda nakon takve obrade se naziva omekšana voda. Omekšavanje vode izvodi se uglavnom primenom tri osnovna postupka: zagrevanjem vode, hemijskim omekšavanjem vode dodavanjem raznih hemikalija kao što su nitrati ili kaustična soda(stariji načini) i omekšavanje vode primenom neutralne jonske izmene (moderniji način). Hemijsko taloženje se sprovodi uglavnom kod većih energetskih sistema, dok se kod malih energetskih sistema i u industriji koriste jonski izmenjivači. Omekšavanje vode sprovodi se kod obrade vode za industrijsku upotrebu, u pripremi rashladne ili napojne vode, te u prehrambenoj industriji proizvodnji piva i bezalkoholnih pića.


Dekarbonizacija vode podrazumeva delimično omekšavanje vode, odnosno uklanjanje soli karbonatne tvrdoće [Ca(HCO3)2 i Mg(HCO3)2]. Dekarbonizacija se može sprovesti toplotnom obradom vode, dodavanjem gašenog kreča u vrućem ili hladnom stanju, te kiselinom ili slabo kiselim jonskim izmjenjivačem, u cilju pripreme vode kod proizvodnje piva i bezalkoholnih pića, te u pripremi napojnih ili rashladnih voda. Uklanjanje ukupne, odnosno karbonatne tvrdoće hemijskim putem podrazumeva primenu sledećih taložnih sredstava: kalcijum hidroksid (gašeni kreč), natrijum karbonat (kalcinirana soda), natrijum hidroksid (kaustična soda), te fosfatne soli.
Jonska izmena
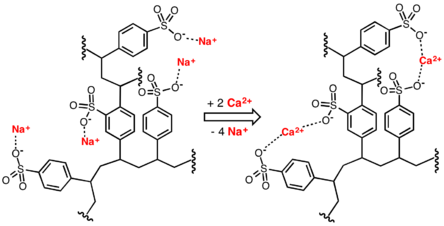
Jonska izmena je postupak koji obuhvata upotrebu jonskih izmenjivača koji mogu vezati jone iz rastvora, a otpuštati jednaku (ekvivalentnu) količinu vlastitih jona. Jonski izmenjivači su uglavnom visokopolimerna jedinjenja(postoje i mineralni) koji imaju svojstvo da vežu jone iz rastvora, a pri tome oslobađaju jednaku količinu istoimeno naelektrisanih jona. Jon jonske smole sadrži različite kopolimere čvrsto vezane u trodimenzionalanu strukturu na koju su pričvršćene jonske grupe. Zavisno od strukture postoje katjonski i anjonski izmjenjivači. Upotrebljavaju se za prečišćavanje različitih rastvora, lekova, omekšavanje ili demineralizaciju vode i drugo.
Jonska izmena je postupak pri kojem se koristi sposobnost određenih materija da jone iz vlastitih molekula zamene za jone iz rastvora. Jonski izmenjivači su nerastvorne visokomolekularne materije (jonske smole), s pozitivnim ili negativnim naelektirsanjem, koje razmenjuju jone bez vidljivih fizičkih promena. Prema hemijskom sastavu jonski izmenjivači mogu biti neorganski ili organski, te prirodni ili sintetički. S obzirom na ulogu dele se na katjonske ili anjonske jonske izmenjivače. Vanjski oblik jonske smole je različit, pa mogu biti u obliku cevi, kuglica, vlakana ili membrane. Različiti zahtevi prečišćavanja otpadne vode primenom jonske izmene pri uklanjanju neželjenih jona iz vode mogu se postići primenom samo jedne vrste jonske smole ili kombinacijom više njih.
Prirodni neorganski alumosilikatni izmenjivači su gline (npr. montmorilonit) i zeoliti (npr. analcit, kabazit), i sintetski gel permutiti (za omekšanje vode). Prirodni organski izmenjivači su npr. ugljenici i celuloza, koji su hidrofilne i porozne prirode, pa je izmena jona brza. Ona može biti neobrađena ili obrađena uvođenjem izmenjivačkih grupa. U modernoj laboratorijskoj praksi prirodni jonski izmenjivači zamenjeni su sintetskim produktima, jonskim smolama koje datiraju negde iz polovine 20. veka. Važni su i sintetski gel izmenjivači dobijeni iz poprečno vezanog dekstrana (Sephadex) ili poliakrilamida (Bio-Gel). To su i molekularna sita. Svi navedeni jonsko-izmenjivački materijali nerastvorni su u vodi, ali mogu izmenjivati vlastite pokretljive protivjone s jonima iz okolnog medija, npr. iz morske vode koja sadrži oko 0,7 mol/dm3 elektrolita.
Tvrdoća vode
Tvrdim vodama se nazivaju vode s visokim, a mekim s niskim sadržajima jona kalcijuma Ca+2 i magnezijumaMg+2. Pri grejanju vode dolazi do remećenja hemijske ravnoteže i izdvajanja taloga:
-
- Ca+2 + CO3–2 => ↓CaCO3↓
- Mg+2 + 2 OH– => ↓ Mg(OH)2↓
U sistemima grejanja, na mestima snažne razmene toplote dolazi do jakog taloženja i stvaranja čvrstih naslaga na zidovima kroz koje se razmenjuje toplota (npr. cevni električni grejači vode u mašinama za pranje veša, cevi zaslona parnih kotlova). Izdvojene naslage ili kamenac svojim dodatnim toplotno-izolacionim delovanjem ometaju razmenu toplote. U početku samo se smanjuje koeficijent korisnog učinka razmenjivača toplote, a najgora je konačna posledica eksplozija parnog kotla. Kako bi se ovo sprečilo, voda se pre punjenja ili dopunjavanja sistema grejanja omekšava taloženjem Ca+2 i Mg+2 jona hemijskim postupcima ili njihovim zadržavanjem u ispunama jonskih izmjenjivača. Hemijsko taloženje se sprovodi uglavnom kod većih energetskih sistema, dok se kod malih energetskih sistema i u industriji koriste jonski izmenjivači. Ako je to potrebno, jonski izmenjivači se izvode u paru (radni/pričuvni), ali, često je dovoljan i samo jedan jonski izmenjivač (etažni sistem centralnog grejanja). U oba slučaja, nakon proticanja određene količine vode jonska smola, kojom je ispunjen jonski izmenjivač, biva zasićena Ca+2 i Mg+2 jonima i mora se obnoviti (regenerirati). Obnova jonskih smola se obavlja s rastvorima hlorovodične kiseline (zamena katjona s H+ jonima) i natrijum hidroksida (zamena anjona s OH– jonima).



Које су последице употребе тврде воде у различитим гранама индустрије?
Kod generatora pare tečna voda prelazi u paru koja se koristi za različite tehnološke potrebe. U tečnoj vodi koja isparava dolazi do povećanja koncentracije rastvorenih jona i porasta njene gustine. Porast gustine vode preko određene granica doveo bi do poremećaja rada generatora pare, te ugušćenu vodu treba blagovremeno ispustiti iz generatora pare (odmuljivanje), što je praćeno značajnim gubicima. Kako bi se ispuštanje vode smanjilo, voda se pre ulaza u kotao priprema postupkom demineralizacije jonskim izmenjivačima.
Vrste tvrdoće vode
Razlikuju se 3 vrste tvrdoće vode:
- stalna ili nekarbonatna tvrdoća vode (za vodu koja u sebi sadrži soli kalcijuma i magnezijuma u obliku sulfata ili nitrata);
- privremena ili karbonatna tvrdoća vode (za vodu koja u sebi sadrži soli kalcijuma i magnezijuma u obliku bikarbonata);
- ukupna tvrdoća vode (stalna + privremena).
Added November 03, 2017 at 5:46pm
by Prof Radovanovic
Title: Појашњење теме


General Document Comments 0





Да ли мислите да је текст разумљив и да су објашњења на месту?





0 archived comments